SOUND Trial Findings and Discussion
The SOUND trial which was recently published in JAMA Oncology concluded that patients with small breast cancer (<2cm) and sonographically normal appearing lymph nodes can be safely spared any axillary surgery whenever the lack of pathological information does not affect the postoperative treatment plan.
This study was designed to evaluate whether omission of SLN surgery in patients with negative axillary ultrasound was noninferior to SLN surgery in terms of 5 year distant disease free survival. While this trial is unlikely to change practice immediately, it is a thought provoking study that will likely generate multidisciplinary discussion. We have highlighted some of the findings and discussion regarding the study for our members below;
- Phase III Randomized Controlled Trial conducted at 18 European hospitals from 2012-2017
- Enrolled patients with Invasive breast cancer up to 2cm, cN0, planning for BCT and XRT who had an axillary US showing no LN involvement on imaging. If doubtful – FNA performed and had to be negative [1406 negative AUS, 57 with negative FNA]
- Patients were randomized to SLN surgery vs no axillary surgery
- Analysis cohort – 1405 women – 708 SLN, 697 no axillary surgery
- Median age 60, Tumor size 1.1 (IQR 0.8-1.5cm), ER+/Her2- disease in 87.8%
- In the SLN group - 13.7% had positive nodes on SLN (5.1% macromets, 8.6% micromets, 2.0% had ≥ 2 positive SLNs, 0.6% had pN2 disease)
- Recommended adjuvant systemic therapy and radiotherapy were similar in the 2 groups
- 20.1% of SLN group and 17.5% of no axillary surgery group received chemotherapy
- 98.0% of SLN group and 97.6% of no axillary surgery received radiation
- 83.3% (593 pts) vs 81.1% (565 pts) had whole breast radiation over 3-5 weeks
- 10.7% (76 pts) vs 10.8% (75 pts) had partial breast radiotherapy
- 3.4% (24 pts) vs 5.6% (39 pts) had intraoperative boost of ELIOT (12 Gy) followed by a hypofractionated course of whole-breast radiotherapy (37.05 Gy in 13 fractions)
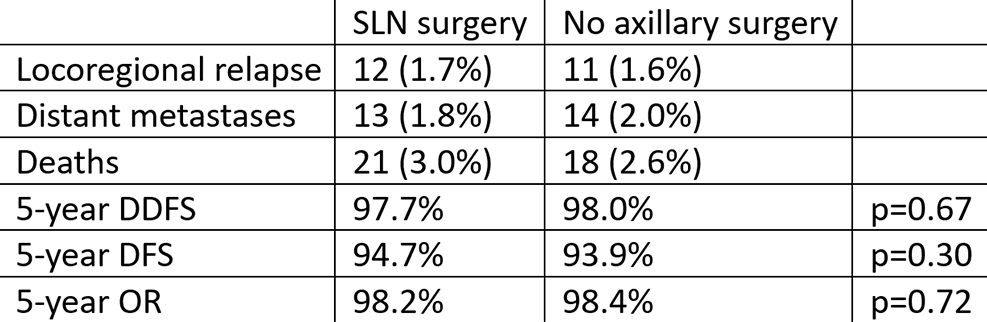
The study authors concluded that patients with patients with small breast cancer with sonographically normal appearing lymph nodes can be safely spared any axillary surgery whenever the lack of pathological information does not affect the postoperative treatment plan.
This study provides further data supporting that axillary sentinel lymph node surgery does not provide therapeutic benefit. In the no axillary surgery group, the cumulative incidence of lymph node recurrences in the axilla was very low (0.4% at 5 years), despite a 13.7% rate of nodal involvement in the SLNB group.
However, SLN surgery likely still has a role in certain patients for staging to guide adjuvant therapies – in particular in young patients where chemotherapy is associated with survival benefit for node positive disease. Furthermore, while adjuvant treatment recommendations in terms of rate of chemotherapy was similar between the two groups, identification of nodal positivity in ER+ breast cancer also influences treatment options in terms of CDK4/6 inhibitor eligibility as well as consideration of extended endocrine therapy (to 10 years).
Many patients are interested in potential for omission of radiation therapy. The trial required radiation, with 90% of patients having whole breast radiation and 10% partial breast radiation. Some of the patients in this trial with small breast cancers aged >65 would be candidates for consideration of omission of radiation. This creates a dilemma regarding de-escalating axillary surgery leading to potential escalation of adjuvant radiation.
It should be noted that tumor grade was not an inclusion/exclusion factor. However, 18% had grade 3 disease. Patients with grade 3 disease have higher likelihood of nodal positivity – should omission of SLN surgery be limited to grade 1 and 2 disease at outset. Especially as grade 3 disease with 1-3 positive nodes would make patients eligible for CDK4/6 inhibitor.
Genomic scores were not included on this trial. Most patients with ER+/Her2- disease (with tumors >1 cm in size) would be considered for genomic testing to guide systemic treatment recommendations.
In summary, multidisciplinary discussion will be important before implementing any changes in practice as a result of the SOUND trial. We look forward to additional data from several other trials evaluating this question over the upcoming years (INSENA, BOOG 2013-08 and NAUTILUS).
Sentinel Lymph Node Biopsy vs No Axillary Surgery in Patients With Small Breast Cancer and Negative Results on Ultrasonography of Axillary Lymph Nodes: The SOUND Randomized Clinical Trial. Gentilini et al. JAMA Oncol. 2023 Sep 21:e233759. doi: 10.1001/jamaoncol.2023.3759

