View the up-to-date information for the 2026 Annual Meeting.

Media Tip Sheet
Contact
Molly McDougall/
Jeanne-Marie Phillips
HealthFlash Marketing
203-977-3333
molly@healthflashmarketing.com
Sharon Grutman
The American Society of Breast Surgeons
877-992-5470
sgrutman@breastsurgeons.org
Download
Additional Notable Research Presented at the 23rd Annual Meeting of the American Society of Breast Surgeons
The following newsworthy abstracts presented at the 23rd Annual Meeting of the American Society of Breast Surgeons (ASBrS) may be of particular interest, in addition to presentations during the Media Press Briefing. Researchers are available for telephone interviews. Onsite media is invited to attend all scientific sessions.
Abstracts
-
Abstract: Intervention to Hepatic and Pulmonary METastases in Breast Cancer Patients: Prospective, Multi-institutional Registry Study-IMET; Protocol MF 14-02
Lead Author: Atilla Soran, MD
University of Pittsburgh, Department of Surgery
Pittsburgh, PA -
Abstract: Survival Outcomes in Patients Following Locoregional Treatment for DCIS: Analysis of the NCDB DCIS Special Study Cohort
Lead Author: Sabrina Wang, MD
Duke University School of Medicine
Durham, NC -
Abstract: Economic Impact of Reducing Re-excision Rates after Breast Conserving Surgery in a Large, Integrated Health System
Lead Author: Jeffery Chakedis, MD
The Permanente Medical Group
Lafayette, CA
ATTRIBUTION TO THE 23rd ANNUAL MEETING OF THE AMERICAN SOCIETY OF BREAST SURGEONS IS REQUESTED IN ALL COVERAGE.
Abstract, Official Proceedings
Intervention to hepatic and pulmonary METastases in breast cancer patients: Prospective, multi-institutional registry study-IMET; Protocol MF 14-02
Authors: Atilla Soran1, Serdar Ozbas2, Beyza Ozcinar3, Arda Isik4, Lutfi Dogan5, Kazim Senol6, Ahmet Dag7, Hasan Karanlik8, Ozgur Aytac9, Guldeniz Karadeniz Cakmak10, Kubilay Dalci11, Mutlu Dogan5, Yavuz Atakan Sezer12, Mustafa Sehsuvar Gokgoz6, Enis Ozyar13, Efe Sezgin14, Breast Health Working Group International
Institutions: 1University of Pittsburgh, Department of Surgery, Pittsburg, PA, 2Ankara Guven Hospital, Department of Surgery, Ankara, Turkey, 3Istanbul University, Istanbul Faculty of Medicine, Department of Surgery, İstanbul, Turkey, 4Medeniyet University, Department of Surgery, Istanbul, Turkey, 5Ankara Oncology Hospital, Department of Surgery, Ankara, Turkey, 6Uludag University Faculty of Medicine, Department of Surgery, Bursa, Turkey, 7Mersin University, Faculty of Medicine, Department of Surgery, Mersin, Turkey, 8Istanbul University, Institute of Oncology, Istanbul, Turkey, 9Baskent University, Department of Surgery, Adana, Turkey, 10Zonguldak Bulent Ecevit University, Department of Surgery, Zonguldak, Turkey, 11Cukurova University, Department of Surgery, Adana, Turkey, 12Trakya University, Department of Surgery, Edirne, Turkey, 13Acibadem Hospital, Department of Radiation Oncology, Istanbul, Turkey, 14 Izmir Institute of Technology, Faculty of Engineering, İzmir, Turkey
Objective: One-fourth of early-stage breast cancer (BC) becomes metastatic at follow-up. Limited metastases represents a clinical state of metastatic disease that is limited in the number of metastatic sites and extent of disease, and amenable to metastasis-directed intervention. The aim of this prospective study is to evaluate intervention to limited metastases in lung and/or liver.
Methods: Luminal A/B and/or HER-2 neu (+) patients with operable lung and/or liver metastases in follow-up after primary BC treatment is completed, and patients who were diagnosed with metastasis after 2014 were included in the study. Demographic, clinical, tumor-specific data, and metastasis detection-free interval (MDFI) were collected. Bone metastasis, in addition to lung and liver metastases, were also included the analysis. Patients were divided into 2 groups according to the treatment modality to metastases - either systemic therapy only (ST) or intervention (IT). The characteristics of the patients were compared with X2 test. Overall survival curves were calculated according to the Kaplan-Meier (KM) (log-rank) method. A multivariable analysis was performed by Cox regression. Statistical significance was defined as a p-value <0.05.
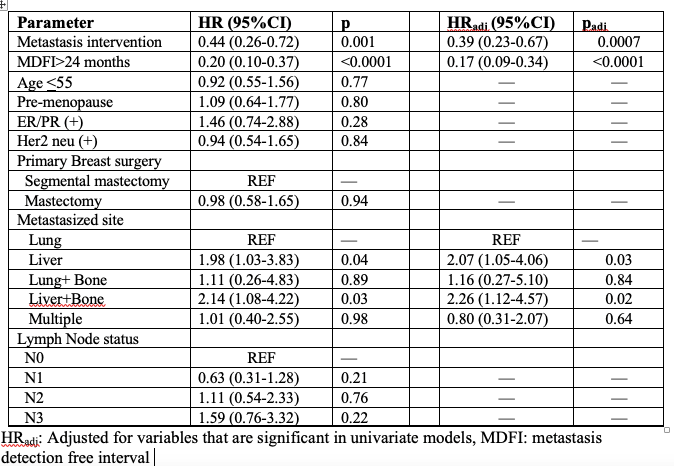
Results: Two hundred patients were enrolled until June 2020. Demographic data were similar between the groups; median follow-up time was 77 (range:55-107) months in IT group (n= 119; 59.5%) and 57 (range:39-84) months in ST group (n=81; 40.5%). Median MDFI was 40 (range:23-70) months and 35 (range:13-61) months, respectively in IT and ST groups (p=0.47). The groups had similar primary tumor and axillary surgery; the majority of them (74%) had axillary lymph node dissection. The majority of the patients had liver metastasis (n=116, 58.0%), and 101 (50.5%) of patients had lung metastases; 17 (8.5%) patients had both lung and liver metastases. Primary tumor was ER/ PR (+) in 150 (75.0%) patients and 64 (32.0%) patients had HER2 neu (+) tumors. Metastatic site surgical resection was done in 64 (32.0%) patients, and 55 (27.5%) patients underwent metastatic ablative interventions. In KM survival analysis, hazard of death (HoD) was 56% lower in the IT group than ST group (HR 0.44: 95% CI 0.44; 0.26-0.72; p=0.001). The HoD was lower in the IT group than ST group regarding age <55 (HR 0.32: 95% CI 0.17-0.62; p=0.0007). In the multivariable cox regression model, HoD was significantly lower in patients who underwent intervention to metastases and who had MDFI >24 months compared to no intervention group and shorter MDFI, but having liver metastases increases the HoD 2 times compared to lung metastases (Table).
Conclusions: Metastasis-directed interventions have reduced the risk of death in patients with limited lung/liver metastases who are amenable to interventions after primary cancer treatment is completed. In the selected group of patients such as luminal A/B, HER2 neu (+) BC, younger than 55 years old, limited metastases to lung and/or liver, and MDFI >24 months, surgical or ablative therapy to metastases should be considered and discussed in the tumor boards.
Abstract, Official Proceedings
Survival outcomes in patients following locoregional treatment for DCIS: Analysis of the NCDB DCIS Special Study cohort
Authors: Sabrina Wang1, Yi Ren2, Terry Hsylop2, Thomas Lynch3, Marc Ryser4, Amanda Francescatti5, Anne McCarthy5, Shelley Hwang6
Institutions: 1Duke University School of Medicine, Durham, NC, 2Duke University Medical Center Department of Biostatistics and Bioinformatics, Duke Cancer Institute, Durham, NC, 3Department of Surgery, Duke University Medical Center, Durham, NC, 4Department of Population Health Sciences, Department of Mathematics, Duke University, Durham, NC, 5American College of Surgeons, Chicago, IL, 6Duke University, Durham, NC
Objective: Ductal carcinoma in situ (DCIS) is a preinvasive diagnosis for which breast conserving surgery (BCS), breast conserving surgery with adjuvant radiation (BCS+RT), and mastectomy are all approved to be guideline-concordant locoregional treatments. BCS without RT has been the least utilized treatment, due in part to concerns regarding potential reduction in breast cancer mortality with this option. Survival endpoints for DCIS have largely been reported from national cancer registries, with important limitations inherent in incomplete or inaccurate data collection. To address these issues, we partnered with the American College of Surgeons Commission on Cancer (CoC) to use primary source documentation as the definitive data source at each site, in order to compare survival outcomes in patients treated for DCIS.
Methods: In this CoC Special Study, a treatment-stratified random sample of patients diagnosed with screen-detected DCIS was selected from the National Cancer Database (NCDB) at 1,330 CoC accredited facilities. A CoC registrar at each site reviewed the original source documentation for each case, for up to 20 cases per site. Detailed diagnosis, treatment, pathology, and follow up information were abstracted and centrally collected. Differences between treatment groups were assessed using the Chi-square test or Kruskal-Wallis test for categorical or continuous variables, respectively. After inverse probability weighting (IPW), a Cox Proportional Hazards model was used to estimate the differences in breast cancer mortality and overall mortality between BCS, BCS+RT, and mastectomy groups.
Results: The final study cohort included 18,983 patients diagnosed with biopsy-confirmed DCIS between 2008 and 2014. Locoregional treatment for DCIS consisted of BCS alone (n=4,236; 22.4%), BCS-RT (n=10,051; 52.9%), or mastectomy (n=4,696; 24.7%). Significant differences between treatment groups included age (p<0.001), race (p=0.0011), DCIS grade (p<0.001), Charlson comorbidity (p<0.0001), hormone receptor status (p<0.001), use of endocrine therapy (p<0.001), insurance (p<0.001), facility type and location (p<0.0001), and DCIS detection method (p<0.0001), and comedonecrosis (p<0.0001). After IPW of variables, a multivariable analysis showed overall survival to be best in BCS+RT [BCS HR 3.38 (2.79-4.09); mastectomy HR 1.39 (1.13 -1.72)]. Importantly however, the multivariable analysis showed no significant differences in breast cancer mortality between treatment groups when compared to BCS+RT [BCS HR 1.87 (0.57-6.10); mastectomy HR 2.60 (0.93 -7.27)].
Conclusions: In a large, well curated dataset of patients treated for DCIS, we found no difference in breast cancer mortality between women treated with BCS, BCS-RT or mastectomy. These results should provide confidence in offering individualized locoregional treatment, including BCS alone, without fear of compromising breast cancer survival.
Abstract, Official Proceedings
Economic impact of reducing re-excision rates after breast-conserving surgery in a large, integrated health system
Authors: Jeffery Chakedis1, Annie Tang2, Alison Savitz3, Liisa Lyon4, Patricia Palacios5, Benjamin Raber6, Brooke Vuong6, Maihgan Kavanagh6, Gillian Kuehner6, Sharon Chang6
Institutions: 1The Permanente Medical Group, Lafayette, CA, 2Department of Surgery, University of California San Francisco, East Bay - Highland Hospital, Oakland, CA, 3The Permanente Medical Group, Walnut Creek, CA, 4Kaiser Permanente Division of Research, Oakland, CA, 5Enterprise Business Services Kaiser Foundation Health Plan, Oakland, CA, 6The Permanente Medical Group, Oakland, CA
Objective: Re-excision after breast-conserving surgery (BCS) is costly for patients and the health care system, but high-level data capturing these costs are limited. A toolbox of methods for reducing re-excision rates have previously been implemented by the American Society of Breast Surgeons. We quantified the association of re-excision after BCS with both direct operating room (OR) costs and utilization of OR time to model expected savings of a re-excision reduction initiative.
Methods: Using institutional databases and the electronic medical record, we performed a retrospective cohort review of all breast cancer patients with BCS between January 1, 2016 and December 31, 2020. Re-excision was defined as an ipsilateral breast operation within 6 months after initial BCS and included both margin re-excision and completion mastectomy. Operating room costs of disposable supplies and implants as well as operative time were calculated. A model was created to simulate a quality improvement program that would decrease the re-excision rate of all surgeons to 20%.
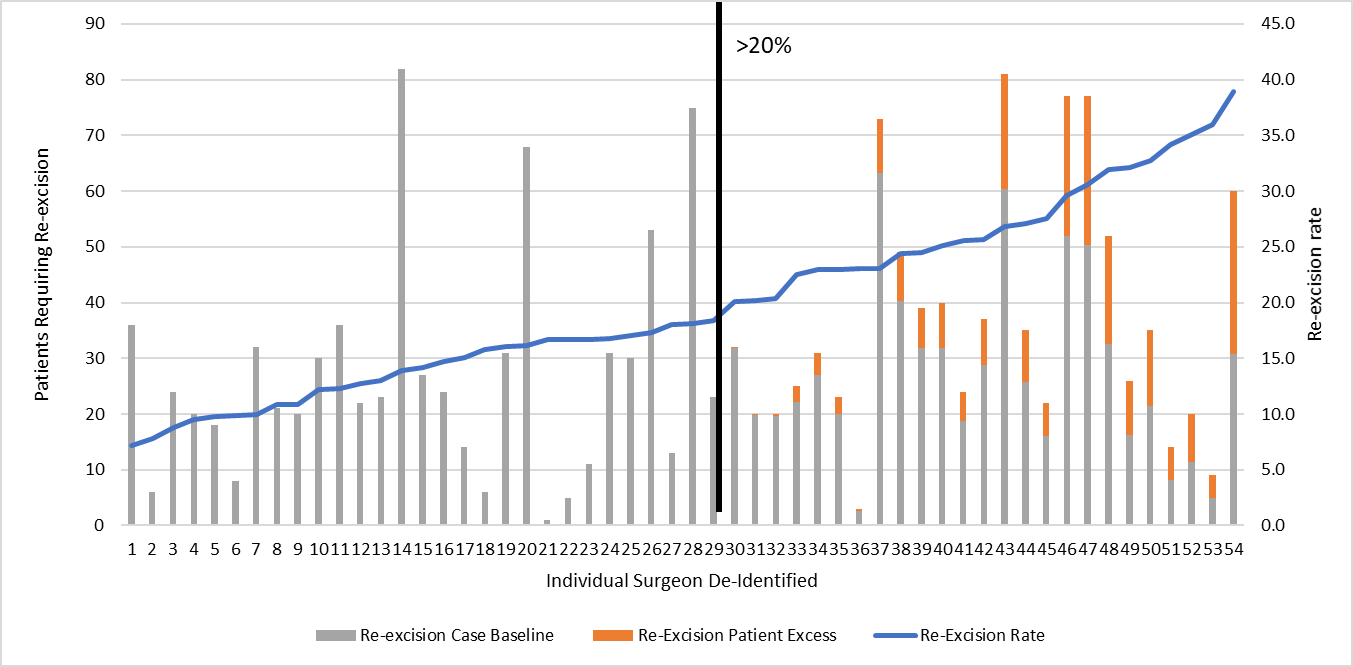
Results: Over the 5-year period, 8,804 patients were treated with BCS, and 1,628 (18.5%) required re-excision. The patients who required re-excision compared to those who did not were similar in age (61 vs. 64 years, p<0.001) but were more likely to have ductal carcinoma in situ (23.7% vs. 15.2%, p<0.001) and had larger tumors (T1+T2 73.2% vs. 83.1%, p<0.001). The 1,828 re-excision procedures included 1,434 (78%) margin re-excisions and 394 (22%) mastectomies. Re-excision costs represented 39% of total OR costs (re-excision -$1,762,193, all procedures - $4,506,519). The cost per patient to complete breast surgery was 4-fold higher for patients requiring re-excision ($1,374 vs. $316, p=0.001). The additional OR costs for margin re-excision ($503, 42% increase) and mastectomy without reconstruction ($657, 125% increase) were lower compared to mastectomy with reconstruction ($8,447, 29x increase). Operative times for the initial lumpectomy were longer in cases that required re-excision (77 vs. 72 minutes, p<0.001). Re-excision operations totaled 1,848 hours and comprised 14% of total OR time (13,030 hours). Margin re-excision were an average 41 minutes (n= 1434) and an average 132 minutes (n=394) for each re-excision mastectomy. The re-excision rate for 54 surgeons varied from 7.2%-39.0%, with 46% (n=25) having a re-excision rate over 20%. Surgeon-specific average costs for each operation varied (BCS $108-$726 and re-excision $116-$4,817) however, and were not associated with surgeon re-excision rates. A model to simulate improving re-excision rates to less than 20% for all surgeons yielded an improved average re-excision rate of 16.2% and would save 236 patients a re-excision. This model predicted a decrease in re-excision operations by 18% (327 operations), OR costs by 14% ($287,534), and OR time by 11% (204 hours).
Conclusions: Re-excision after BCS represents 39% of direct OR costs and 14% of OR time, which has a significant impact on health system economics. Our health system has initiated a margin re-excision reduction project utilizing routine cavity shave margins. Modest reductions in surgeon re-excision rates may lead to significant OR cost and time savings.